leap forward in the fight against cancer
PanTher’s technology unleashes the inherent power of cancer-fighting drugs by enabling continuous, high-dose treatment exclusively at the site of a tumor.
Our lead product, PTM-101, is now in a
Phase 1b study.
Technology Convergence with PanTher’s innovative cancer drug development platform


to tumors can be leveraged via minimally invasive procedures that have become broadly used in cancer care.
new generation of long-lasting, high-dose, local chemotherapeutic cancer treatments
More drug where you want it
Over 100 times increase in drug concentration at the tumor site compared to systemic administration
Less drug where it’s not needed
Limited, if any, systemic exposure which enables favorable tolerability
Long-lasting Therapeutic effect
Continuous release of therapeutic levels of drug at the tumor site over a significantly extended duration of treatment (weeks or months)

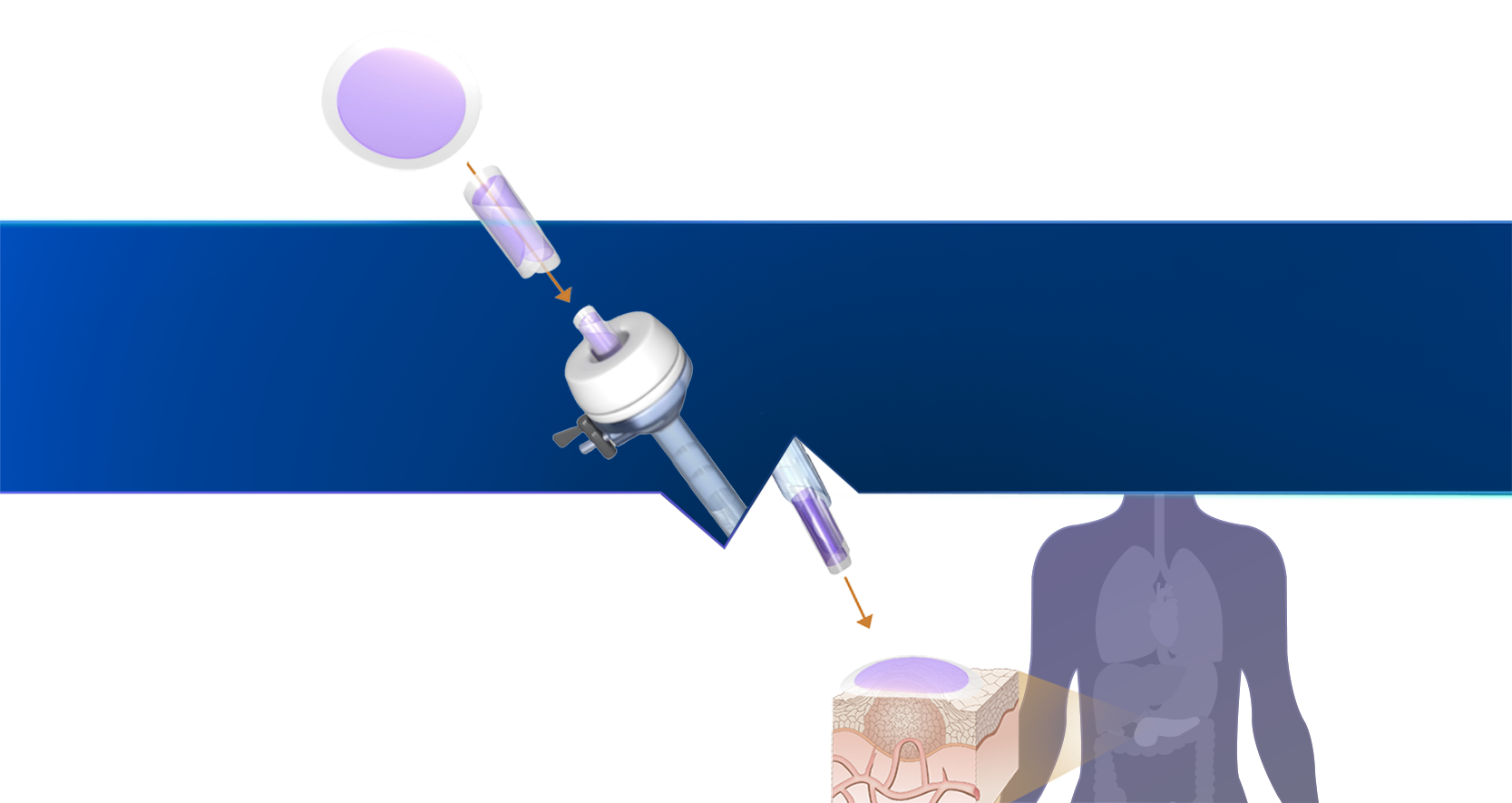
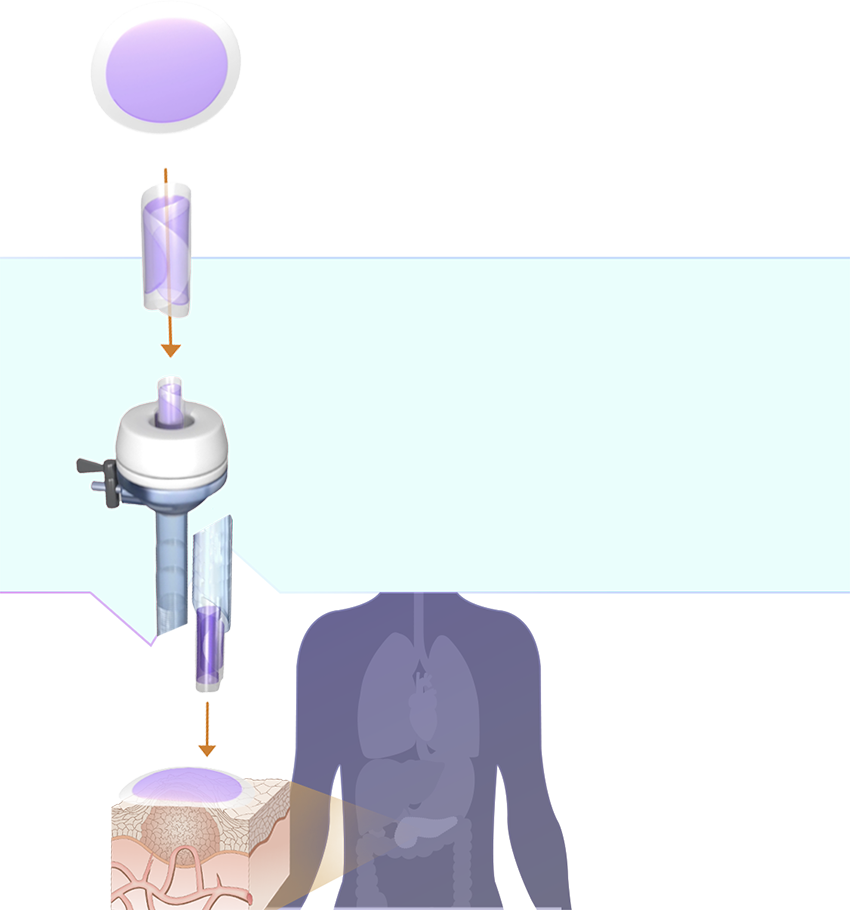
Proprietary technology platform
Our SagittariTM platform enables us to create drug products that are optimized for dose and duration and engineered into a shape that can be easily integrated into existing, interventional oncology procedures.
Lead product candidate
Our lead product candidate, PTM-101, is in ongoing clinical development for the treatment of pancreatic cancer.
See PTM-101 results from first-in-human clinical study presented at AACR 2024
Emerging pipeline opportunities
Based on the broad capabilities of our Sagittari platform, we are exploring a number of pipeline opportunities.